The biopharmaceutical industry must break with existing manufacturing paradigms if it is to reduce the cost of biological medicines and ensure they are accessible to patients around the world. The industry’s next generation of production facilities will use innovative approaches to minimize clean room space utilization, reduce the footprint of facilities and lower production costs.
In order to meet the global demand for biological medicines in these small and agile productions facilities, intensified manufacturing platforms are being developed that allow very high productivity to meet late phase clinical and commercial demand. These platforms utilize mammalian cell hosts in perfusion bioreactors linked to continuous downstream trains. Cell culture productivities have reached a point where a bioreactor can sustain a volumetric productivity of more than 2 g/L/day. This, coupled with the development of low-cost media and advancement in process automation technologies around the bioreactor, has translated to an ability for companies to operate a single-use bioreactor uninterrupted for over 15 days and execute harvest and Protein A capture on a continuous basis.
Optimizing Continuous Biomanufacturing Operating Conditions
Engineers at Just-Evotec Biologics were challenged to find the optimum operating conditions to enable the lowest production costs irrespective of facility mass output. To achieve this aim, they developed process models for our continuous process contained within a J.POD® facility.
J.POD facilities apply modular cleanroom pod technology arranged in a controlled, non-classified ballroom to minimize the cleanroom footprint of operations that would have previously taken place in a large ballroom. Media and buffer preparations, cell expansion, upstream, downstream and post viral are all housed in separate pods. The design minimizes fixed utility infrastructure and instead relies on single-use continuous upstream and downstream operations.
Figure 1 shows the relationship between Cost of Goods Manufactured (COGM) of a therapeutic antibody, volumetric productivity and culture duration. The results indicated that COGM is strongly inversely correlated to volumetric productivity between titers of 0.5 and 3.0 g/L/day, as shown by the sharp drop in COGM with increased volumetric productivity. The inverse correlation is not as strong when comparing COGM and culture duration, which is likely because most of the cost reduction benefits are attained when the first few kilograms of product are manufactured (e.g., because of the high cost of shared downstream disposables). Marginal increases in culture duration will generate higher amounts of product and allow the production of metric tonne-quantities of antibody but are accompanied by a proportional increase in media and downstream buffer costs.
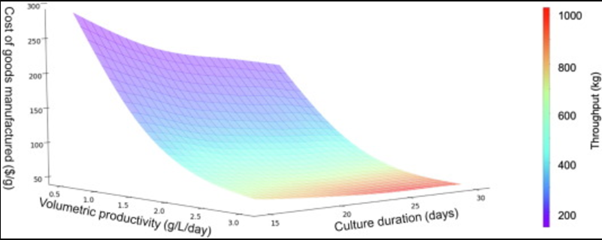
Figure 1. Sensitivity plot illustrating Cost of Goods Manufactured (COGM) versus bioreactor design variables. Color coding was used to layer in throughput as a measure of the three variables studied.
Expanding Access to Life-Saving Medicines
Just-Evotec Biologics has two facilities in North America that are operational and manufacturing biological medicines for clients. Our European facility will be brought online in Toulouse, France in late-2024.
These facilities in two geopolitically stable locations will provide our customers with additional supply chain security. They feature our intensified manufacturing platform allowing the agile and low-cost production of biopharmaceuticals.
The cost-modelling described in this article shows the potential for the J.POD facilities to be capable of delivering metric-tonne quantities of biological medicines for late phase clinical and commercial manufacturing. Furthermore, it illustrates how the COGM decrease dramatically as titers increase so that extremely low production costs can be achieved. We believe that this provides an opportunity to reduce the overall costs of biological medicines and will allow patients around the world greater access to these life-saving medicines.
