Orphan drugs are defined by regulatory authorities as diseases that affect fewer than 200,000 patients in the US, or no more than five in 10,000 people in the EU. These diseases are often genetic and therefore lifelong conditions, typically affecting patients from very early age on.
While financial incentives and flexible regulatory framework present a wealth of opportunities for developers, the complexities around developing drugs for small patient populations can present significant additional challenges for sponsors. But what are the challenges facing developers of drugs for orphan diseases beyond financial and regulatory issues, and how can these be overcome?
A key challenge is understanding the regulatory and financial framework for orphan drugs in different regions. The following table provides an overview of the European, US and Japanese markets: 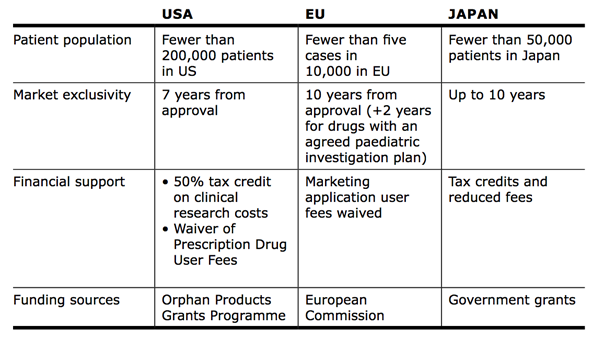
Another major challenge is the lack of information that is often known about individual conditions and their disease mechanism. This, in turn, makes it very difficult to assess the right endpoints for clinical trials. In addition, the success of clinical trials in orphan diseases is also hampered by the identification of countries with a sufficient number of study participants and suitable study centres with the capabilities to conduct the required type of trial and ensure proper patient retention throughout the trial. Therefore, although orphan drugs might seem a safe and attractive bet at first glance, the implementation of successful drug development programs can be tricky.
The support of an experienced orphan drug development partner can significantly increase the chances of success. Building on a wealth of long-term orphan drug development expertise, Evotec´s tightly integrated approach and culture of close collaboration ensures that development challenges can be solved, and innovative products can be delivered at demanding timelines. By implementing the right strategies and putting careful de-risking plans in place, it is possible to overcome the challenges associated with orphan drug development to put safe and effective medicines in the hands of the patients who need them.
Evotec is the right partner to support orphan drugs product development because the Company has GMP facilities approved both for clinical and commercial batches manufacturing well suited for the small amounts of active ingredient and drug product required in this field.
Find out more about Evotec´s orphan drug development and manufacturing capabilities:
API Capabilities
Development Manufacturing
Read our Whitepaper
