Reaching IND stage is a major milestone for biopharmaceutical companies. For decades, the biotechnology industry has struggled to align complex functions towards the goal of getting to the clinic. The process is long, expensive, resource straining, and risky due to high attrition rates.
Recent industry benchmark data shows that neither the costs nor timelines of drug discovery have improved significantly in recent years - regardless of digital solutions and AI. Including the cost of attrition, it takes benchmark companies approx. $75 m just to achieve a single regulatory tox study start and still around 5.5 years to go from a target to an FGLP or IND.
Reaching IND Faster - A Significant Competitive Advantage
Evotec’s data shows that its integrated processes have led to high success rates and enabled reaching the IND milestone at around half the cost and in about 30% less time when compared with the above-mentioned benchmark. The approach has been validated by its own R&D activities and is increasingly demanded by its partners, too.
The reason is simple: time saving is very important, as the potential to reach IND 18 months faster than the competition adds real value in a competitive marketplace. This is especially true in a highly connected world, where many players are pursuing the same or similar scientific concepts.
The Solution: INDiGO
In order to provide a standardised, yet versatile solution to advance compounds to IND as a service for its partners and clients, Evotec has established INDiGO as a unique integrated, accelerated IND-enabling platform to reduce tech transfer times and costs. The platform is well-suited for a broad range of indications.
INDiGO offers interdisciplinary integration and expert coordination of all drug development activities under one roof. This enables unmatched timelines from candidate nomination to regulatory submission. The programme is led by experienced, dedicated project managers and drug development professionals, who are responsible for seamless knowledge transfer across disciplines, while maximising the quality of the overall development package and ensuring the highest quality standards.
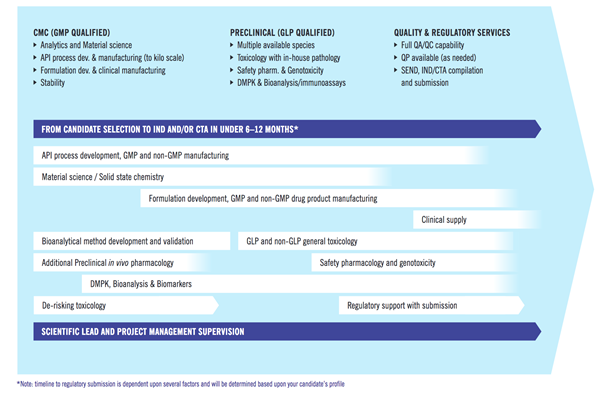
INDiGO Highlights at a Glance
• More than 40 different functions across multiple disciplines
• Managed on an operational level by more than 100 experienced drug development professionals
• Industry leading timelines and excellent track record of on-time delivery
• > 35 completed programmes in the last 5 years
• High rate of client retention after first successful INDiGO programme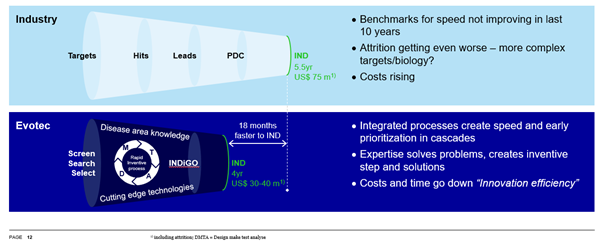
Learn more about:
