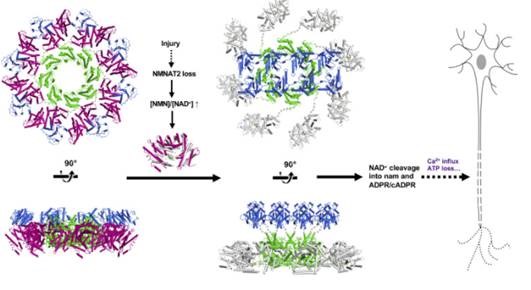
SARM1 is a NADase whose action triggers the destruction of axons and development of novel SARM1 inhibitors which enables the prevention or delay of neurodegenerative disorders. This paper identifies the binding site for the SARM1 agonist NMN and reveals the structure of full-length SARM1 as elucidated by cryo-electron microscopy (cryo-EM). The structure of apo SARM1 was revealed as an octameric ring, held in an autoinhibitory state by the separation of the active TIR domain at the rim of the ring. The elucidation of autoinhibition release and the identification of the NMN binding site within the autoinhibitory ARM domain opens a path to inhibition of SARM1 via stabilisation of its inactive form. These studies were possible through the close co-ordination of the new cryo-EM team at Evotec, Abingdon with Disarm and academic collaborators in Australia and the USA.
Proposed SARM1 activation mechanism: