From Batches to Brilliance: The Benefits of Continuous for Commercial Manufacturing
As we covered in the first blog of this series, biotherapeutics sponsor companies face numerous challenges in process development and manufacturing. This largely comes down to the overwhelming reliance on fed-batch processes in the biologics industry, which bring lengthy production timelines, process risks, and supply chain vulnerabilities. These issues contribute to the high cost structure of biologics and limit the accessibility of these vital therapies worldwide.
Continuous manufacturing is a rapidly emerging alternative production process to fed-batch processing. Biological products are produced in an uninterrupted flow, resulting in a steady and consistent output of product. This approach addresses many of the challenges with fed-batch systems, including:
- Reduced process costs and lower cost of goods manufactured (COGM)
- Scalability and adaptability to fluctuating demands
- Fewer process and scalability risks
Read on as we explore these benefits of continuous manufacturing for commercial manufacturing, describing how they can address the high cost structure associated with biologics, and help to meet the rising global demand for these life-saving therapies.
Cost reduction
Continuous manufacturing can reduce the COGM by up to 75% compared to traditional fed-batch processes (1), while also achieving 10-fold higher productivity (Figure 1).
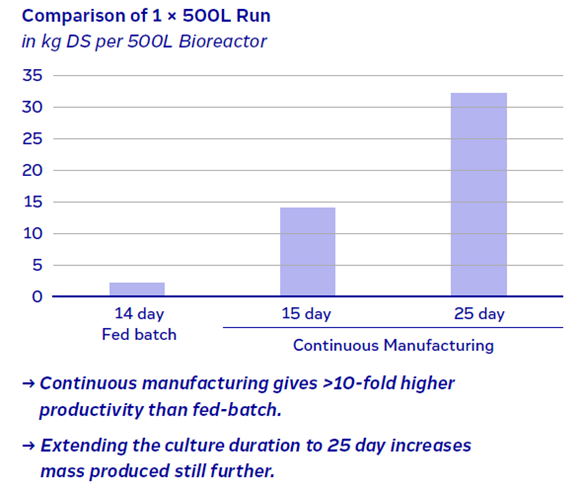
Figure 1: Productivity of a fed-batch process compared with a continuous approach.
This is partly achieved through workflow automation, which minimizes labor costs while improving production efficiency (Figure 2). Additionally, compared with fed-batch units that require large facilities to store product intermediates, continuous operations are highly intensified and have a much smaller facility footprint, further reducing operational costs. Continuous operations also enable manufacturers to optimize resource use and reduce waste, leading to additional cost savings.
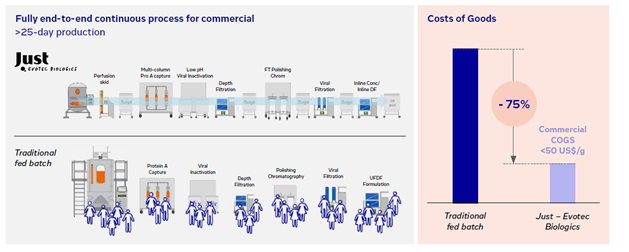
Figure 2: Unit operations and labor resources needed to run a continuous process compared to traditional fed-batch process
Scalability and adaptability
A continuous approach allows for a much more agile process, driven by greater scalability and adaptability. For instance, J.POD® biomanufacturing facilities from Just – Evotec Biologics support throughput from less than 10 kg to over 2,000 kg per year of protein-based biologics including mAbs and biosimilars. Production can be rapidly scaled up by increasing bioreactor numbers and extending run times with intensified continuous manufacturing technology. This enables manufacturers to adjust production levels quickly in response to market demand.

Figure 3: How Just – Evotec Biologics’ platform steady-state intensified continuous manufacturing process can be scaled
Reduced risk
Continuous manufacturing reduces risks by ensuring consistent product quality. A DoE approach can be used to fine tune product quality attributes (PQAs) during development and advanced monitoring in production can maintain process consistency. This consistency is crucial for reducing regulatory risks and ensuring the efficacy and safety of biologics.
This innovative manufacturing approach also enhances supply chain and financial security. With a smaller facility footprint and reduced operational costs, the financial risk for sponsor companies is substantially reduced. The modular design of facilities like J.POD allows for rapid deployment and scaling, mitigating geopolitical disruptions and financial risks associated with traditional fed-batch processes.
Unravelling the blueprint for success
Transitioning to continuous manufacturing for commercial supply, in modular facilities like J.POD, offers a robust, cost-effective, and adaptable solution to address the unmet demand for biologics globally. Yet despite the clear benefits of continuous manufacturing, many manufacturers have yet to make the transition from fed-batch systems. This is often due to a lack of understanding of regulatory uncertainties, deployment pathways, and process development activities.
Just – Evotec Biologics leverages over a decade of expertise in continuous manufacturing to help clients convert their fed-batch processes to its continuous manufacturing platform ready for commercial production. Commercial supply runs with its continuous manufacturing platform can be performed within one of its J.POD biomanufacturing facilities. These modular, scalable facilities are designed to offer the following benefits:
- Maximized yields and cost efficiency – J.POD facilities utilize intensified continuous perfusion culture supporting high cell densities and product yield. The facilities also minimize operational costs through workflow automation, intensified bioprocessing, optimized resource use, and more.
- Rapid deployment and advanced agility – J.POD facilities are built using modular cleanroom pods that can be swiftly deployed and assembled. This design allows for flexible and scalable manufacturing capacity, enabling the facility to adapt quickly to changing production needs.
- Risk resilience – With established facilities in the US and Europe, J.POD mitigates the risk of geopolitical or supply disruptions. These facilities are standardized in design and operation, allowing for seamless process transfer between sites.
Discover the full benefits of J.POD facilities
References
1. Garcia, F.A. and Gefroh, E. Reducing biopharmaceutical manufacturing costs through continuous processing in a flexible J.POD® facility. Drug Discovery Today. (2023); 28 (7): 103619. https://doi.org/10.1016/j.drudis.2023.103619
