Katie Haughan, Drug Transporter Sciences Team, Cyprotex
Drug transporters play a pivotal role in drug-drug interactions (DDI’s), as such regulatory bodies such as the FDA and EMA recommend the study of specific transporters that are known to cause clinical DDI’s. One of these recommendations for orally administrated drugs, unless waivered based on the BCS classification, is the victim (substrate) potential at Breast Cancer Resistance Protein (BCRP) due to evidence that inhibition of intestinal BCRP in DDI can increase the absorption and therefore exposure of sensitive substrate drugs such as rosuvastatin and topotecan. Pharmacogenetics also need to be considered when developing drugs that are BCRP substrates as this can impact the BCRP expression levels between individuals and within the global population and can therefore result in differing pharmacokinetic profiles. Polymorphisms associated with BCRP may or may not impact on the expression or functionality of the transporter, however the prevalence of these can vary dependent on the ethnicity. One example that is of importance for understanding the pharmacogenetic impact is BCRP SNP C421A, which is associated with lower BCRP protein expression, and leads to the ABCG2-Q141K polymorphism which has a higher frequency in Japanese and Chinese populations compared to Caucasian populations [Birmingham et al. 2015, Hua et al. 2012]. As a result, the plasma levels of BCRP substrates such as rosuvastatin in these populations is increased due to greater absorption and therefore this would need to be considered for Cmax estimates and dosage strategies. Differences in BCRP expression amongst the global population can be a result of many factors such as ethnicity, genetic polymorphisms and disease states.
The industry gold standard, and regulatory recommendation, for BCRP substrate identification is the use of a polarised cell monolayer system to determine the bidirectional flux of the investigational drug in the absence and presence of a selective inhibitor. To do so there are two cell lines which are favoured across the industry; BCRP over expressing transfected Madin-Derby Canine Kidney cell line (MDCK-BCRP), or the immortalised colorectal adenocarcinoma cell line Caco-2, both of which differentiate and polarise allowing for the expression on a range of proteins and display the in vivo like characteristics such as tight junctions.
In order to select which cell line to utilise in these regulatory studies there are many aspects to consider, each cell line comes with its own advantages and disadvantages and can favour the outcome of certain classes of compounds. Whilst MDCK-BCRP has advantages over Caco-2 such as short culturing times and higher expression of the transporter of interest, there is a risk that data interpretation may be clouded due to an intrinsic limitation of the MDCK cell line which, for efflux transporter substrate determination, may result in false negatives. This potential error in the reported classification can have implications downstream as being a substrate may reduce the oral absorption and bioavailability of the drug, which may result in therapeutic dose not being achieved. Whilst transfected MDCK cells have been used for many years in order to assess an investigational drug’s BCRP substrate status, it’s applicability may be limited due to the cells lacking expression of the relevant basolateral uptake transporters that allow polar substrates to enter the cell and in turn interact with BCRP transporter on the apical membrane. Without this uptake mechanism, and in combination with the compounds poor permeability, the basolateral to apical flux would be negligible resulting in an efflux ratio (ER) less than 2 (B-A/A-B). The initial classification by the FDA is dependent on this ratio with a BCRP substrate having an efflux ratio greater than 2.
Whilst Caco-2 cells have a considerably longer culture time compared to that of MDCK cells, they have the benefit of being able to correctly identify the efflux compounds that rely upon the interplay between apical and basolateral transporters. Organic solute transporter alpha (OST-α) is expressed on the basolateral membrane of epithelial cells in the small intestine, kidney, liver, and colon amongst other organs aswell as on Caco-2 cells, and this trans-membrane protein allows passive facilitative transport of endogenous and exogenous molecules across the membrane, including polar structures. A primary function of OST-α is the transport of bile acids, and therefore lends itself to the transport of other polar substrates such as the prototypical BCRP substrates rosuvastatin and estrone 3-sulfate across the membrane and into the cell. Once inside the cell the compound then has access to the binding site of the BCRP transporter and the opportunity to be effluxed if it is indeed a substrate. For cells that lack this uptake mechanism, such as MDCK-BCRP, polar compounds have restricted entry to the cell and do not have the opportunity to be effluxed regardless of if they are a substrate of BCRP or not.
Rosuvastatin is one of the most widely prescribed statins and implicated in BCRP DDI’s due to its victim classification. Rosuvastatin is a polar compound with a logP of 0.13 and therefore has low permeability and is dependent upon uptake mechanisms to enter cells such as MDCK. The flux of rosuvastatin, apical to basolateral, basolateral to apical and the subsequent efflux ratios can be seen in Table 1 [Li et al. 2012] for three cell lines. The B-A secretory apparent permeability (and derived efflux ratio) is significantly higher (and therefore more sensitive) in Caco-2 cells compared to that in the two MDCK cell lines even though the protein expression of the BCRP transporter in Caco-2 cells would be considerably lower than in the transfected MDCK-BCRP cells, demonstrating the crucial requirement of the basolateral uptake mechanism that is present in Caco2, but absent in MDCK-BCRP, to facilitate the interaction of rosuvastatin with intracellular BCRP on the apical membrane. This factor needs to be taken into consideration when it comes to assessing the efflux potential of an investigational drug using MDCK-BCRP cells.
Table 1: Bidirectional (apical-to-basolateral (A-B) & basolateral-to-apical (B-A)) apparent permeability (Papp) and efflux ratios for rosuvastatin in different cell test systems [1]
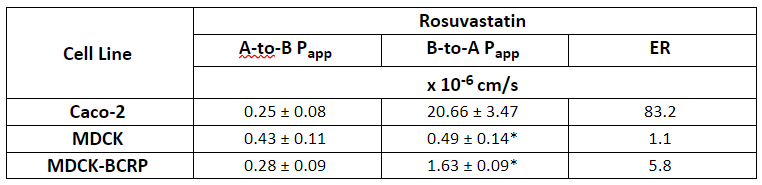
*P<0.001, significance level of the difference from the B-to-A transport in Caco-2
As shown the use of Caco-2 cells for BCRP substrate identification removes a complication seen in MDCK-BCRP cells that is dependent on the physicochemical properties of the compound, however Caco-2 cells come with their own complexities. As Caco-2 are human derived cells they express a range of endogenous human transporters including human P-glycoprotein (P-gp). BCRP and P-gp have a similar substrate profile and therefore the efflux seen in Caco-2 cells may be due to BCRP, Pgp efflux or a combination of both. In order to decipher between the two main efflux transporters a selective P-gp inhibitor such as verapamil can be added to the test system buffer to chemically knockdown P-gp activity. The residual efflux is then considered to be due to BCRP transport however this can be confirmed using a selective BCRP inhibitor, fumitremorgin C (FTC) as shown in Table 2.
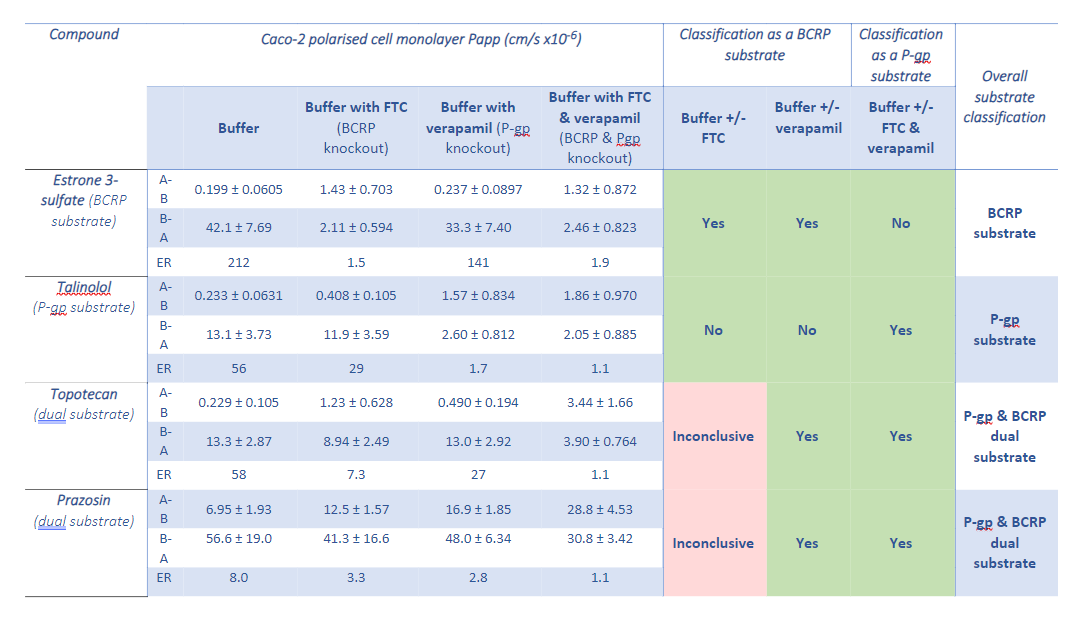
Table 2: Bidirectional (apical-to-basolateral (A-B) & basolateral-to-apical (B-A)) apparent permeability (Papp) and efflux ratios for test compounds and the substrate classifications given per assay condition
Choosing the right in vitro cell test system for BCRP substrate identification is critical for achieving the correct classification. The two gold-standard approaches have their own pros and cons, each of which can be mitigated if these test system limitations are understood. If a chemical series has a reasonable degree of lipophilicity then MDCK-BCRP should correctly identify a substrate of BCRP. However, as the industry moves towards more metabolically stable molecules and chemical series that have similar low intrinsic permeability and polarity to the clinically relevant BCRP substrate rosuvastatin, the use of Caco-2 cells would be required for the correct identification of BCRP substrates in order to avoid false negatives.
References
- Birmingham BK, Bujac SR, Elsby R, et al. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol. 2015 Mar;71(3):341-55.
- Li J, Wang Y, Zhang W, Huang Y, Hein K, Hidalgo IJ. The role of a basolateral transporter in rosuvastatin transport and its interplay with apical breast cancer resistance protein in polarized cell monolayer systems. Drug Metab Dispos. 2012 Nov;40(11):2102-8.
- Hua, W.J., Hua, W.X. and Fang, H.J, The Role of OATP1B1 and BCRP in Pharmacokinetics and DDI of Novel Statins. Cardiovascular Therapeutics,. 2012, 30: e234-e241.
