The industry gold-standard in vitro cell based test system for studying Breast Cancer Resistance Protein (BCRP) or P-glycoprotein (P-gp) transporters is the polarized cell monolayer system using either Caco-2 cells or MDCK-MDR1 cells (Elsby et al., 2022), grown on semi permeable membrane inserts to form a brush border membrane barrier separating two experimental compartments (apical and basolateral) of equivalent pH (7.4). Apparent permeability (Papp, cm/s x10-6) of the test article is determined in the apical to basolateral (A-B) and the basolateral to apical (B-A) direction and from this an efflux ratio is calculated (B-A Papp/A-B Papp). An efflux ratio greater than 2 which is reduced by greater than 50% with a corresponding reduction in B-A Papp in the presence of a reference inhibitor (Atkinson et al., 2022) indicates the test article is a substrate of the efflux transporter being investigated. The bidirectional Papp values and subsequent efflux ratios of the known P-gp substrate quinidine, determined in polarized MDCK-MDR1 cell monolayers across a concentration range is summarized in Table 1 (left) and demonstrates that in the MDCK-MDR1 polarized monolayer test system quinidine has been determined to be a substrate of the P-gp transporter.
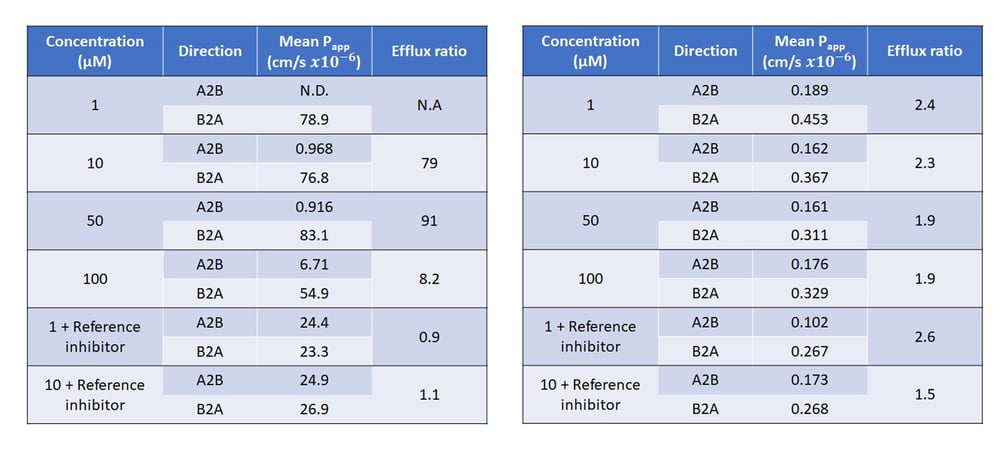
Table 1: Mean Papp values and corresponding efflux ratios of quinidine (left) and N-methyl quinidine (right) across MDCK-MDR1 monolayer cells in the presence and absence of reference inhibitor cyclosporin A
Despite polarized monolayers being the industry gold standard test system for studying P-gp and BCRP interactions, there are limitations and risk of inconclusive results and false negatives if the test article being studied exhibits low Papp indicating inherently low passive membrane permeability. This is due to the physico-chemical characteristics of the test article limiting cellular entry required to access the efflux transporter on the apical membrane. This is true for the quinidine oxidative metabolite, N-methyl quinidine (NMQ). The presence of the methyl group results in lower lipophilicity and therefore passive permeability without diminishing its ability to bind P-gp. As summarized in Table 1 (right) bidirectional Papp values for the more polar NMQ are very low and although efflux ratios determined are around two, a 50% reduction in efflux ratio with corresponding decrease in B-A Papp is not observed in the presence of inhibitor therefore the result is inconclusive at best, or at worst could be interpreted as not a substrate of the P-gp transporter.
Where assessment of a test article in polarized monolayer cells indicates a compound has inherently low passive permeability, membrane vesicles have the membrane orientated “inside out”, this results in the intracellular binding site of the transporter being positioned on the outside of the vesicle (outwardly facing), therefore compounds do not need to cross a membrane in order to interact with the transporter. As such for efflux transporters such as P-gp or BCRP, substrates will be actively taken up into the vesicle and, due to poor permeability, will remain inside the vesicle for quantification. As demonstrated in Table 2, using P-gp membrane vesicles as an in vitro test system for P-gp substrate identification, NMQ is clearly demonstrated to be, and subsequently correctly identified as, a substrate of the P-gp transporter.

Table 2: Mean uptake rate and corresponding uptake ratios of N-methyl quinidine using P-gp expressing membrane vesicles in the presence of ATP or AMP in the absence and presence of the reference inhibitor cyclosporin A. Figure: Mean uptake rate of N-methyl quinidine into P-gp expressing membrane vesicles in the presence of ATP or AMP in the absence and presence of the reference inhibitor (I) cyclosporin A
Furthermore, if data from P-gp or BCRP substrate identification studies in polarized cell monolayers indicate a compound has inherently low passive permeability then any P-gp or BCRP inhibition data generated in the same in vitro test system should also be scrutinized and a follow up inhibition study in membrane vesicles considered, particularly if no inhibition was observed in the cells.
Whilst membrane vesicles have their advantages over polarized cell monolayers as a test system to identify P-gp or BCRP substrates that are poorly permeable, it is not advisable to utilize membrane vesicles as a first-choice test system. This is because for lipophilic test articles such as quinidine, sufficient passive permeability allows the substrate to move freely across the membrane and would not be trapped within the vesicle lumen, therefore there is a risk of inconclusive results and false negatives.
In summary, polarized cell monolayers are the gold standard for studying P-gp and BCRP interactions as indicated in regulatory guidances and as such should be the first choice of test system for identifying P-gp or BCRP substrates and inhibitors. However, if results indicate the compound has inherently low passive permeability then P-gp or BCRP expressing membrane vesicles should be considered as an alternative follow up in vitro test system to mitigate against the clinical implications of false negatives.
References:
Atkinson, H., Mahon-Smith, K. and Elsby, R. (2022) ‘Drug permeability and transporter assessment: Polarized Cell Lines’, The ADME Encyclopedia, pp. 401–412. doi:10.1007/978-3-030-84860-6_142.
Elsby, R. et al. (2022) Studying the right transporter at the right time: An in vitro strategy for assessing drug-drug interaction risk during drug discovery and development. Expert Opin Drug Metab Toxicol 18(10):. 619–655. doi:10.1080/17425255.2022.2132932.
