Check out Evotec's infographic on Aerodynamic Particle Size Distribution and learn how our dedicated team of scientists has the right experience in analytical inhalation to ensure accuracy and reliability.
Check out Evotec's infographic on Aerodynamic Particle Size Distribution and learn how our dedicated team of scientists has the right experience in analytical inhalation to ensure accuracy and reliability.
Tags: Infographics, Formulation & CMC, IND Enabling Studies/Preclinical Development
Watch our first virtual roundtable on continuous manufacturing of biologics. Together with industry thought-leaders we aim to discuss current trends in biomanufacturing in this series. For this session we invited Brian Kelley, SVP Process and Product Development at Vir Biotechnology and Randal Bass, EVP Process Design and Biotherapeutic Operations at Just-Evotec Biologics. They discuss "How real is continuous manufacturing and what are the challenges?".
Want to learn more? Reach out to us at info@evotec.com
Tags: Videos & Webinars, Biologics
Date: March 18-20
Location: Barcelona, Spain
Attending
Tags: Events, Evotec, Just Evotec Biologics
Date: February 3-7
Location: Boston, MA, USA
Attending
Date: January 19
Location: Foster City, CA, USA
Attending
Watch the 20 minute interview with Jon Gunther, VP Business Development Just-Evotec Biologics, at Biomanufacturing World Summit 2023. For a complete table of content regarding Jon's discussion, please see below.
Table of Contents
0:00 min – 01:50 min
Intro + Discussion on biomanufacturing costs and making biotherapeutics more affordable and accessible to patients worldwide
01:51 min –5:06 min
Emergence of continuous manufacturing of biologics to reduce cost of goods manufacturing (COGM)
5:07 – 7:00 min
Switching from fed-batch to continuous manufacturing, quality requirements and stance of the FDA
7:01 – 08:32 min
Timelines for switching to continuous manufacturing
08:33 –10:42 min
Pharma 4.0 / Using state-of-the-art technology incl. lights-out manufacturing approach
10:43 – 13.42 min
Highly flexible manufacturing approach with Just-Evotec Biologics’ J.POD cGMP manufacturing facilities in North America and Europe
13.43 – 16:30 min
What should commercial partners observe in terms of cost, quality, scale-up and how this process can be de-risked
16:31 – 17:30 min
J.POD’s flexible and de-risked manufacturing approach when going from late-stage clinical to commercial supply
17:31 – 19:44
Our flexible partnership model incl. options like with our tech partnership with Sandoz for biosimilars development and commercial manufacturing
19:45 – end
Closing comments, get in touch with us!
Tags: Videos & Webinars, Biologics
The biopharmaceutical industry must break with existing manufacturing paradigms if it is to reduce the cost of biological medicines and ensure they are accessible to patients around the world. The industry’s next generation of production facilities will use innovative approaches to minimize clean room space utilization, reduce the footprint of facilities and lower production costs.
In order to meet the global demand for biological medicines in these small and agile productions facilities, intensified manufacturing platforms are being developed that allow very high productivity to meet late phase clinical and commercial demand. These platforms utilize mammalian cell hosts in perfusion bioreactors linked to continuous downstream trains. Cell culture productivities have reached a point where a bioreactor can sustain a volumetric productivity of more than 2 g/L/day. This, coupled with the development of low-cost media and advancement in process automation technologies around the bioreactor, has translated to an ability for companies to operate a single-use bioreactor uninterrupted for over 15 days and execute harvest and Protein A capture on a continuous basis.
Engineers at Just-Evotec Biologics were challenged to find the optimum operating conditions to enable the lowest production costs irrespective of facility mass output. To achieve this aim, they developed process models for our continuous process contained within a J.POD® facility.
J.POD facilities apply modular cleanroom pod technology arranged in a controlled, non-classified ballroom to minimize the cleanroom footprint of operations that would have previously taken place in a large ballroom. Media and buffer preparations, cell expansion, upstream, downstream and post viral are all housed in separate pods. The design minimizes fixed utility infrastructure and instead relies on single-use continuous upstream and downstream operations.
Figure 1 shows the relationship between Cost of Goods Manufactured (COGM) of a therapeutic antibody, volumetric productivity and culture duration. The results indicated that COGM is strongly inversely correlated to volumetric productivity between titers of 0.5 and 3.0 g/L/day, as shown by the sharp drop in COGM with increased volumetric productivity. The inverse correlation is not as strong when comparing COGM and culture duration, which is likely because most of the cost reduction benefits are attained when the first few kilograms of product are manufactured (e.g., because of the high cost of shared downstream disposables). Marginal increases in culture duration will generate higher amounts of product and allow the production of metric tonne-quantities of antibody but are accompanied by a proportional increase in media and downstream buffer costs.
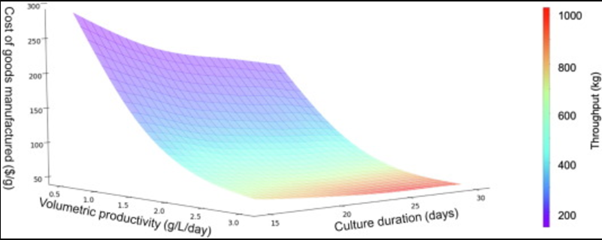
Figure 1. Sensitivity plot illustrating Cost of Goods Manufactured (COGM) versus bioreactor design variables. Color coding was used to layer in throughput as a measure of the three variables studied.
Just-Evotec Biologics has two facilities in North America that are operational and manufacturing biological medicines for clients. Our European facility will be brought online in Toulouse, France in late-2024.
These facilities in two geopolitically stable locations will provide our customers with additional supply chain security. They feature our intensified manufacturing platform allowing the agile and low-cost production of biopharmaceuticals.
The cost-modelling described in this article shows the potential for the J.POD facilities to be capable of delivering metric-tonne quantities of biological medicines for late phase clinical and commercial manufacturing. Furthermore, it illustrates how the COGM decrease dramatically as titers increase so that extremely low production costs can be achieved. We believe that this provides an opportunity to reduce the overall costs of biological medicines and will allow patients around the world greater access to these life-saving medicines.
Date: February 28 - 29
Location: Dublin, Ireland
Attending
Tags: Events, Evotec, Just Evotec Biologics
Patients around the world have limited or restricted access to biopharmaceutical medicines. Reducing production costs while still maintaining high quality standards will help increase the affordability of biologics and ensure more patients benefit from these life-saving medicines.
Traditional biomanufacturing facilities have failed to deliver biopharmaceutical products with sufficiently low Cost of Goods Manufactured (COGM) to allow greater patient access. These facilities have been built with fixed capacity and a focus on large-scale fed-batch manufacturing. Scaling-up processes to large-scale fed-batch manufacturing facilities involves considerable risk, resource, and upfront costs. Such facilities often lack flexibility which limits the products that can be produced within them and can leave valuable production assets idle for periods of time.
Manufacturing costs link directly to capacity utilization and product demand. There is a historical precedent within the biopharmaceutical industry of operating with excess capacity but we must recognise this comes with a financial penalty. We must address this challenge if we are to respond to global healthcare emergencies, changes in the way healthcare systems are managed and greater demand for global access to biotherapeutics.
Continuous Bioprocessing Platforms and Modular Facility Designs
The industry needs new flexible biomanufacturing concepts to quickly react to market fluctuations and achieve a higher predictability of costs. Modern biopharmaceutical productions facilities use building and manufacturing technologies, such as modular construction, to minimize clean room space utilization and reduce footprints. They allow faster speed to market with a lower upfront capital investment and are readily expandable when product demand is better understood. Continuous manufacturing platforms can be integrated into these facilities for low-cost bioprocessing using mammalian cell hosts in perfusion bioreactors linked to continuous downstream trains. Production costs remain low irrespective of facility mass output, the product quality attributes are consistent and manufacturing footprints are minimized.
Just-Evotec Biologics has developed a low-cost manufacturing facility design utilizing modular cleanroom pod technology that we call J.POD®. The J.POD facility design features individual pre-fabricated cleanroom pods arranged in a controlled, non-classified ballroom to minimize the cleanroom footprint of operations that would have previously taken place in a large ballroom. Media and buffer preparations, cell expansion, upstream, downstream and post viral are all housed in separate pods. The design minimizes fixed utility infrastructure and instead relies on single-use continuous upstream and downstream operations.
Biomanufacturing Facility Cost Comparisons
We developed process models for a fed-batch process in a traditional stainless-steel facility, a fed-batch process in a single-use facility and three continuous processes in a J.POD facility. The models were created using the Biosolve software (Biopharm Software Ltd) to show the benefit of the J.POD facility design on the COGM of an antibody biologic. We used Net Present Cost (NPC) to compare scenarios. NPC estimates cash flows by computing operational costs and discounting over time using a capital parameter. It does not include revenues in the accounting of cash flows and assumes capital costs are sunk costs incurred at the beginning of the project.
Figure 1 shows the expected costs from operating the different facility types and assumes their throughput increases at a rate of 250 kg/year up until a peak value. The range selected was representative of market demands for typical biopharmaceutical manufacturing facilities. Jumps in the NPC correspond to points when the capacity of a facility is reaches and new builds are needed.
We can draw the following conclusions from the results.

Driving Up Access to Biotherapeutic Medicines
We believe that modern biomanufacturing facilities must have smaller processing spaces, higher production throughputs and lower production costs. Modelling shows how our J.POD facilities have the lowest initial build and operating costs as well as the ability to control operating costs. These facilities are outperforming older manufacturing platforms in terms of cost and utilization. They are becoming an essential component of strategies for reducing costs and driving up access to biotherapeutic medicines.
Tags: Articles & Whitepapers, Blog, Biologics
Cardiotoxicity remains one of the most reported adverse drug reactions that lead to drug attrition during pre-clinical and clinical drug development. Drug-induced cardiotoxicity may develop as a functional change in cardiac electrophysiology (acute alteration of the mechanical function of the myocardium) and/or as a structural change, resulting in loss of viability and morphological damage to cardiac tissue.
Our latest work, published in Expert Opinion on Drug Metabolism & Toxicology, is now available to download.