Tags: Medicinal Chemistry, Women's health, Articles & Whitepapers, Hit & Target ID/Validation, Immunology & Inflammation
Tags: Medicinal Chemistry, Articles & Whitepapers, Immunology & Inflammation
Tags: Posters, Sample Management
The idea of targeting messenger RNAs for therapeutic purposes to shut off the translation of proteins dates back to 1978 when researchers discovered the first antisense oligonucleotide, an oligonucleotide complementary to the viral RNA of Rous sarcoma virus. It was capable of binding to the viral mRNA and thereby inhibiting viral replication and protein synthesis.
Meanwhile, a lot of short single-stranded antisense oligonucleotides made from natural or chemically modified DNA and RNA nucleotides have been developed. They all are designed to modify gene expression not only for inhibiting protein synthesis but also for altering RNA and/or reducing, restoring, and modifying protein expression through multiple molecular mechanisms such as splicing. Suppression of target expression is accomplished by binding of the antisense oligonucleotide to the target (pre)mRNA followed by degradation of this (pre)mRNA.
The pharmacology of targeted antisense therapy has provided the basis for translating it to the clinic. Chemical modifications of the oligonucleotides have enhanced the specificity, affinity and efficacy and reduced side effects. Optimisation of delivery and improved resistance to breakdown by nucleases, leading to precisely defined half-life, also have been accomplished. These improvements have brought research from bench to clinic. Additionally, the use of antisense oligonucleotides against long noncoding RNAs (lncRNAs), small interfering RNAs (siRNAs), micro RNAs (miRNAs), and ribozymes have also demonstrated preclinical and clinical responses in the treatment of deadly diseases like cancers.
Antisense oligonucleotides fill a position that is not or only insufficiently addressed by more traditional approaches. As an example, they can address targets undruggable by conventional strategies, e.g. targets that do not have a function or surface that can interact with small molecules or targets that are inaccessible for antibodies. They can also address challenging targets, e.g. targets that need to be addressed selectively as they possess high structural homology to other targets that should not be triggered to avoid unwanted side-effects.
Moreover, they have unique and well-characterized biodistribution and pharmacokinetics and can be manipulated by conjugation to targeting moieties. Also, there are many different administration routes, systemic treatments as well as local (e.g. intrathecal, intravitreal or inhalation) or ex vivo approaches to target cells in the cell therapy setting, e.g. to accomplish a transient target knockdown to improve the manufacturing process, the safety of the cell product or its efficacy.
Several antisense drugs have been approved, most against rare diseases such as Batten disease, cytomegalovirus retinitis, Duchenne muscular dystrophy, or spinal muscular atrophy. As of 2020, more than 50 antisense oligonucleotides were in clinical trials, including over two dozen in advanced clinical trials (phase II or III).
Evotec has considerable expertise in antisense therapy and in 2020 entered into a strategic partnership with antisense specialist Secarna Pharmaceuticals GmbH & Co. KG to build a co-owned antisense drug pipeline based on Secarna’s proprietary LNAplus™ platform. This third generation of LNAs allows to switch off gene expression of single disease-underlying genes in the cell.
The partnership aims to address a number of targets and complex indications, to establish a pipeline of co-owned antisense oligonucleotide therapies and to licence candidates to biopharmaceutical companies.
Learn more:
Reaching IND stage is a major milestone for biopharmaceutical companies. For decades, the biotechnology industry has struggled to align complex functions towards the goal of getting to the clinic. The process is long, expensive, resource straining, and risky due to high attrition rates.
Recent industry benchmark data shows that neither the costs nor timelines of drug discovery have improved significantly in recent years - regardless of digital solutions and AI. Including the cost of attrition, it takes benchmark companies approx. $75 m just to achieve a single regulatory tox study start and still around 5.5 years to go from a target to an FGLP or IND.
Reaching IND Faster - A Significant Competitive Advantage
Evotec’s data shows that its integrated processes have led to high success rates and enabled reaching the IND milestone at around half the cost and in about 30% less time when compared with the above-mentioned benchmark. The approach has been validated by its own R&D activities and is increasingly demanded by its partners, too.
The reason is simple: time saving is very important, as the potential to reach IND 18 months faster than the competition adds real value in a competitive marketplace. This is especially true in a highly connected world, where many players are pursuing the same or similar scientific concepts.
The Solution: INDiGO
In order to provide a standardised, yet versatile solution to advance compounds to IND as a service for its partners and clients, Evotec has established INDiGO as a unique integrated, accelerated IND-enabling platform to reduce tech transfer times and costs. The platform is well-suited for a broad range of indications.
INDiGO offers interdisciplinary integration and expert coordination of all drug development activities under one roof. This enables unmatched timelines from candidate nomination to regulatory submission. The programme is led by experienced, dedicated project managers and drug development professionals, who are responsible for seamless knowledge transfer across disciplines, while maximising the quality of the overall development package and ensuring the highest quality standards.
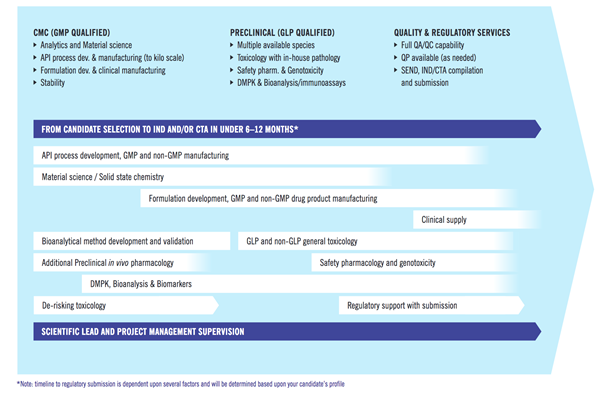
INDiGO Highlights at a Glance
• More than 40 different functions across multiple disciplines
• Managed on an operational level by more than 100 experienced drug development professionals
• Industry leading timelines and excellent track record of on-time delivery
• > 35 completed programmes in the last 5 years
• High rate of client retention after first successful INDiGO programme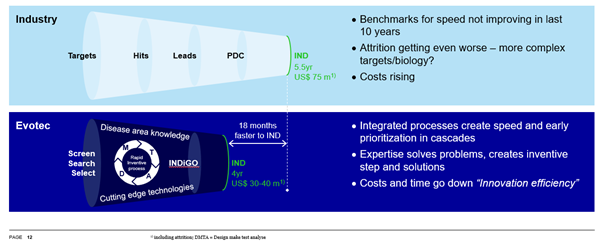
Learn more about:
Cell Painting sounds like art for biologists, and indeed it is a tool that leads to colourful and often very aesthetic images. However, it is a serious and, above all, very useful technology. It provides researchers with a multi-parameter, image-based description of the cell response to any condition that perturbs the cell, e.g. the treatment with a compound, a silencing via siRNA, or CRISPR engineering.
Basically, it (https://www.nature.com/articles/nprot.2016.105) is a morphological profiling assay that multiplexes six fluorescent dyes, imaged in five channels, to reveal eight broadly relevant cellular components or organelles. Cells are plated in multi-well plates, exposed to the treatments to be tested, stained, fixed, and imaged on a high-throughput microscope. Next, automated image analysis can be used to identify individual cells and to measure up to 1,700 morphological features (size, shape, texture, intensity, etc) to produce a rich profile that enables the detection of subtle phenotypes.
Profiles of cells treated with different methods or compounds can be compared to suit many goals, such as identifying the phenotypic impact of chemical or genetic changes, grouping compounds and/or genes into functional pathways, and identifying signatures of disease. The assay offers single-cell resolution and is complementary to the Connectivity Map (https://clue.io/cmap), which characterizes cell population responses to perturbation using transcriptomics.
Evotec has implemented a robust Cell Painting workflow to allow for the study of several thousands of compounds, using automated process for cell treatment and labelling, image analysis, data processing and quality control. The Company now uses Cell Painting to support hit triage at the end of a High Throughput Screening in order to select series with optimised phenotypic characteristics, for example to avoid major off-target effect or keep some degree of biological diversity.
To further improve sensitivity of the assay, Evotec is currently testing different approaches:
Tags: Blog, In vitro Biology