Earlier this year, Evotec hosted a complimentary webinar, ‘INDiGO-Select: profiling and selecting your optimal clinical development candidate’.
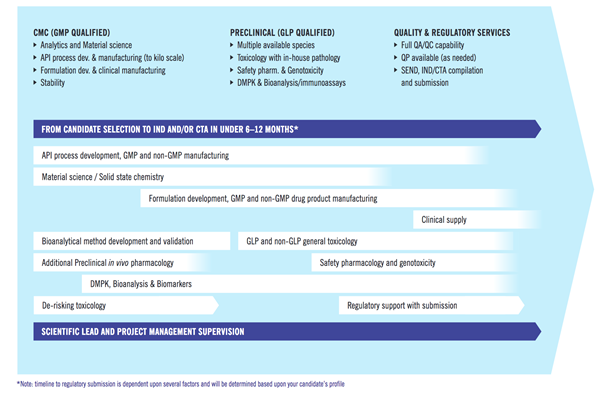
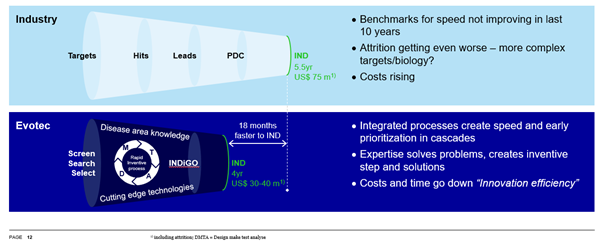
INDiGO-Select focuses on better understanding (or increasing the knowledge) of the lead molecule chemistry, its physical-chemistry properties and preclinical DMPK and safety profile, helping to minimise the risk of failure during transition into the subsequent development stages. Evotec’s Manager, Integrated Development Programmes, Sabrina Pagliarusco and Senior Scientific Project Leader, Federico Tosini, take the audience on a journey starting with the war on attrition and a deeper dive into the importance of having a well-designed approach to de-risking. A relatively small investment at the candidate selection stage allows early identification of potential developability liabilities and challenges which consequently allow for a quicker reaction, at a lower cost.
Evotec’s approach to integrated solutions in drug development – INDiGO-Select and INDiGO – are then highlighted. The INDiGO-Select package is never the same for the candidate as it depends on the data that is generated during the discovery phase. To identify any gaps and technical risks, the key areas of focus are the chemistry and pharmaceutical properties, DMPK, the PK/PD relationship and preliminary safety assessment. The next stage – INDiGO - then accelerates early drug candidates into the clinic by reducing time from nomination to regulatory submission. The advantage is that this program can be customised based on data generated during the select and be conducted at a unique site while integrated, so all actions can be performed under the same roof.
Two case studies that used the INDiGO-Select package are then highlighted. The first focuses on low bioavailability, where the client – a small Biotech – has a therapeutic target in neurodegenerative diseases. The activities performed and data presented highlight the impact that an INDiGO-Select model can have on the progress of IND-enabling activities. A second example looks at chemistry and preclinical PK variability. For this particular case study, the objective was to increase the knowledge on the candidate profile and its developability, reducing the risks of later failure in the full development phase. In this instance, the compound was characterised further and, thanks to the data generated, some potential issues were identified to be considered and monitored during the customised development phase.
Choosing the INDiGO-Select model has a number of benefits including enhanced quality of selected drug candidates, increased probability of success later in development and an overall reduction of development costs and project timelines.
Discover more about INDiGO-Select by streaming this insightful webinar from our experts now!
STREAM THE WEBINAR